A groundbreaking, collaborative initiative designed to: unite leading international industry associations, spearhead global regulatory innovation, and drive digital transformation.
With an ambitious focus on accelerating data exchange and advancing the realization of a fully digital, single, global dossier, the Forum aims to identify policy solutions and advocacy approaches to modernize and streamline regulatory interactions across the life sciences ecosystem.
Key discussion topics include:
- Establishment of global data standards
- Cloud-based data exchange
- Collaborative ways of working
- Strategies to reduce global cycle times
- Relevant legislation and policies
Global Presence
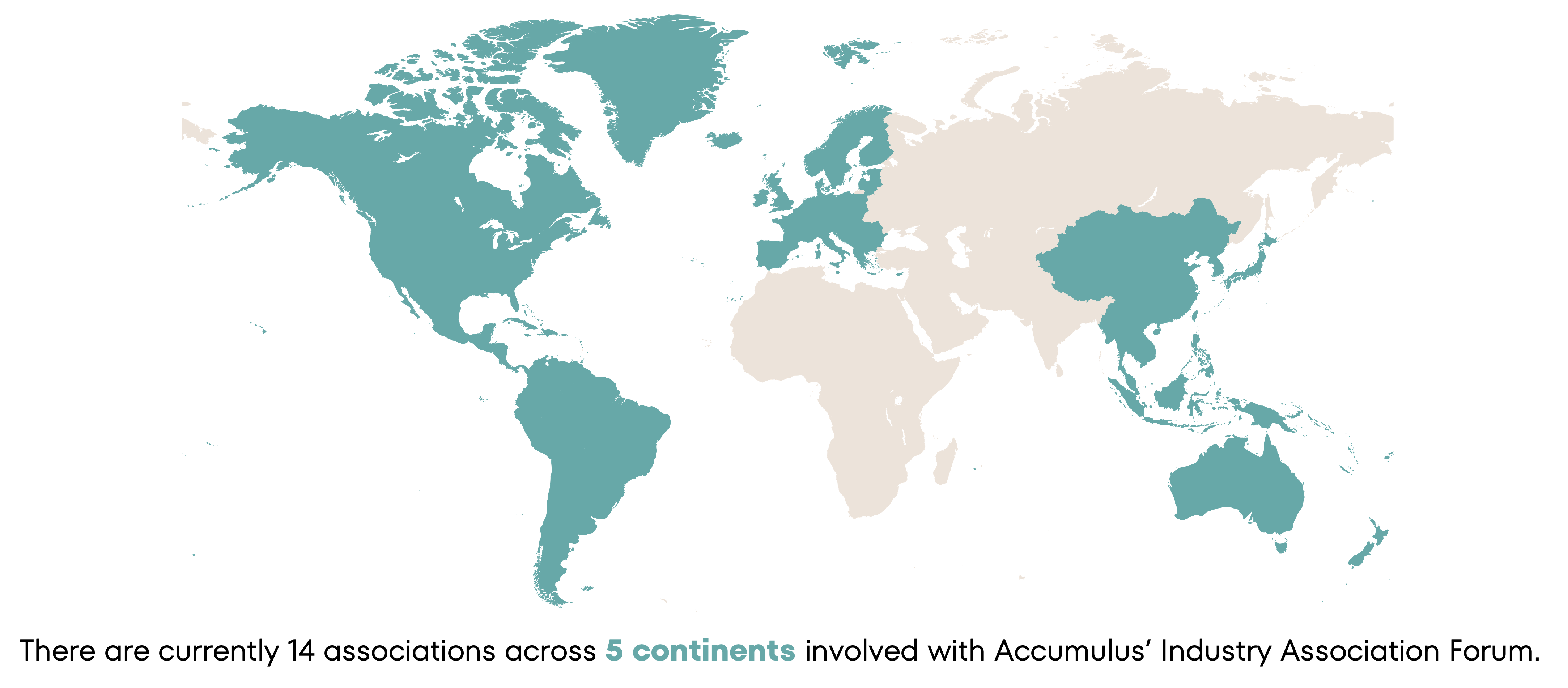
* Participation in the Forum is voluntary and does not serve as a form of endorsement of Accumulus as an organization or of the Accumulus platform.
Influence Change
Shape the future of global, regulatory collaboration, driving impactful advancements to benefit patients around the world


Lead Innovation
Participate in discussions on ways to leverage cutting-edge technology to shorten cycle times and to accelerate critical therapies
Share Your Expertise
Contribute your unique insights, alongside other industry associations, in quarterly meetings designed to propel the industry forward



What Participation
Looks Like

Attend quarterly, virtual meetings

Provide strategic feedback

Engage in dialogue

Drive innovation and share your expertise
Join the Industry Association Forum!

Connect
Reach out to start a conversation today ([email protected])

Select
Select representatives from your organization

Participate
Participate in strategic discussions and be part of a digital transformation





